Arrhenius-Exponential Model: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
{{Reference Example|ALTA_Reference_Examples_Banner.png|ALTA_Reference_Examples}} | {{Reference Example|ALTA_Reference_Examples_Banner.png|ALTA_Reference_Examples}} | ||
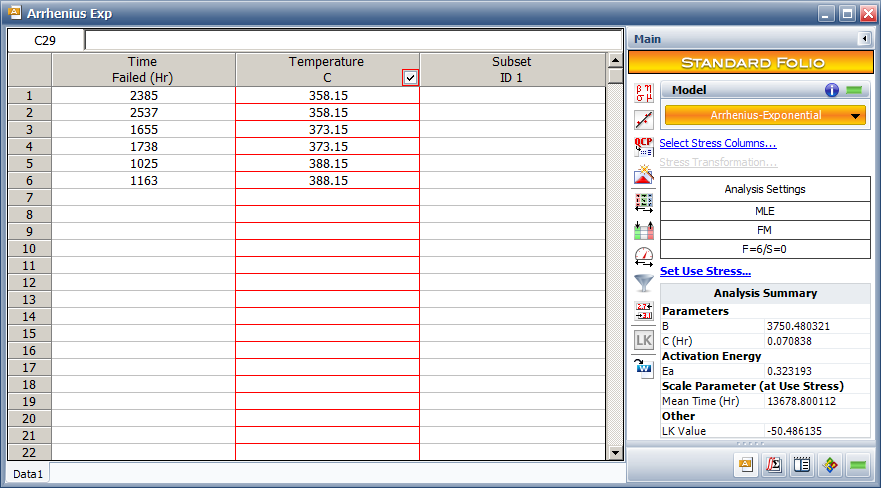
This example | This example compares the results for the Arrhenius-exponential model. | ||
{{Reference_Example_Heading1}} | {{Reference_Example_Heading1}} | ||
| Line 33: | Line 32: | ||
{{Reference_Example_Heading3}} | {{Reference_Example_Heading3}} | ||
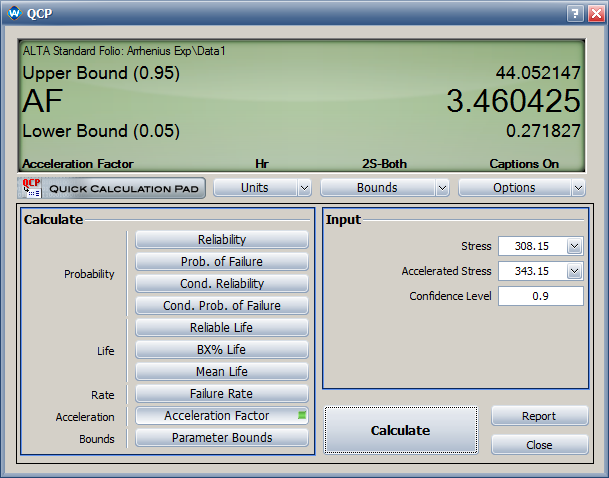
For this data set, the Arrhenius life stress relationship with an Exponential distribution is used. The estimated activation energy value is <math>\,\!E_{a}=0.323</math>. The acceleration factor from 70°C to 35°C is 3.5. | For this data set, the Arrhenius life-stress relationship with an Exponential distribution is used. The estimated activation energy value is <math>\,\!E_{a}=0.323</math>. The acceleration factor from 70°C to 35°C is 3.5. | ||
{{Reference_Example_Heading4|ALTA}} | {{Reference_Example_Heading4|ALTA}} | ||
In ALTA, the temperature values are converted from | In ALTA, the temperature values are converted from Celsius to Kelvin. | ||
[[image:Arrhenius Exp_Folio.png|center]] | [[image:Arrhenius Exp_Folio.png|center]] | ||
Revision as of 15:08, 13 June 2014
ALTA_Reference_Examples_Banner.png